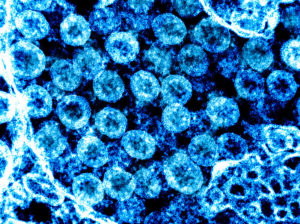
Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: National Institute of Allergy and Infectious Diseases, NIH
Unquestionably good news: virus testing is becoming more available.
Medical research companies, reports James Freeman in the WSJ, are finding ways to confront cornonavirus head-on.
Denise Roland and Peter Loftus report in the Journal:
A new, high-speed coronavirus test has been granted emergency clearance by the Food and Drug Administration, the latest effort to expand capacity to diagnose the fast-spreading pathogen.
The test was developed by diagnostics giant Roche Holding and is designed to run on the company’s automated machines, which are already installed in more than 100 laboratories across the U.S. It will be available immediately.
It is only the third coronavirus diagnostic to receive emergency-use authorization from the FDA, following a test developed by the Centers for Disease Control and Prevention and one from the New York State Department of Health.
What Can Happen when Researchers Urgently Focus on a Single Crisis
An “emergency team” of Roche scientists started work on a new test as soon as China shared the genetic sequence of the virus in January.
It normally takes years for a new test to be developed and approved, according to Roche Diagnostics Chief Executive Thomas Schinecker.
P.S. In the Age of Coronavirus: a Homemade Throat Sanitizer.
P.P.S. If you can’t find hand-sanitizer, you can make your own.
If you’re willing to fight for Main Street America, click here to sign up for the Richardcyoung.com free weekly email.